Hepatitis B Information for Health Care Providers
CCDPH urges healthcare providers to continue to educate parents on the importance of the hepatitis B birth dose and the full 3-dose vaccine series for all babies born in the U.S. CCDPH supports IDPH guidance and the continued use of objective, science-based recommendations. IDPH Guidance for Healthcare Providers
Summary of 2023 HBV screening and testing recommendations
CDC recently published updated recommendations for hepatitis B virus (HBV) screening and testing. Screening refers to testing people not known to be at increased risk for exposure to HBV. Testing refers to conducting serologic tests of people with symptoms or who are identified to be at increased risk for exposure to HBV.
Adults
CDC recommends screening all adults aged 18 years and older for hepatitis B at least once in their lifetime using a triple panel test. To ensure increased access to testing, anyone who requests HBV testing should receive it regardless of disclosure of risk. Many people might be reluctant to disclose stigmatizing risks.
The triple test includes hepatitis B surface antigen (HBsAg), antibody to hepatitis B surface antigen (anti-HBs), and total antibody to hepatitis B core antigen (total anti-HBc). Prior guidance recommended a single test for hepatitis B surface antigen (HBsAg). Any periodic follow up testing can use tests as appropriate based on the results of the triple panel. For information on interpreting test results see: Interpretation of Hepatitis B Serologic Test Results (cdc.gov).
Infants
CDC recommends testing infants born to HBsAg positive people for HBsAg and anti-HBs seromarkers.
Pregnant people
CDC recommends HBV screening for hepatitis B surface antigen (HBsAg) for all pregnant people during each pregnancy, preferably in the first trimester, regardless of vaccination status or history of testing. Pregnant people with a history of appropriately timed triple panel screening without subsequent risk for exposure to HBV (i.e., no new HBV exposures since triple panel screening) only need HBsAg screening.
People at increased risk
CDC recommends testing susceptible people periodically (regardless of age), who have ongoing risk for exposures and while risk for exposures persists. This includes:
- People with a history of sexually transmitted infections or multiple sex partners
- People with hepatitis C infection or a history of hepatitis C virus infection
- People incarcerated or formerly incarcerated in a jail, prison, or other detention setting
- Infants born to HBsAg-positive people
- People born in regions with HBV infection prevalence of ≥2%
- US born people not vaccinated as infants whose parents were born in geographic regions with HBsAg prevalence of >8%
- People who inject drugs or have a history of injection drug use
- People with HIV infection
- Men who have sex with men
- Household contact or former household contacts of people with known HBV infection
- Needle-sharing or sexual contacts of people with known HBV infection
- People on maintenance dialysis, including in-center or home hemodialysis and peritoneal dialysis
- People with elevated liver enzymes
- Those who have never been infected with HBV and either did not complete a hepatitis B vaccine series per ACIP recommendations or who are known to be vaccine non-responders.
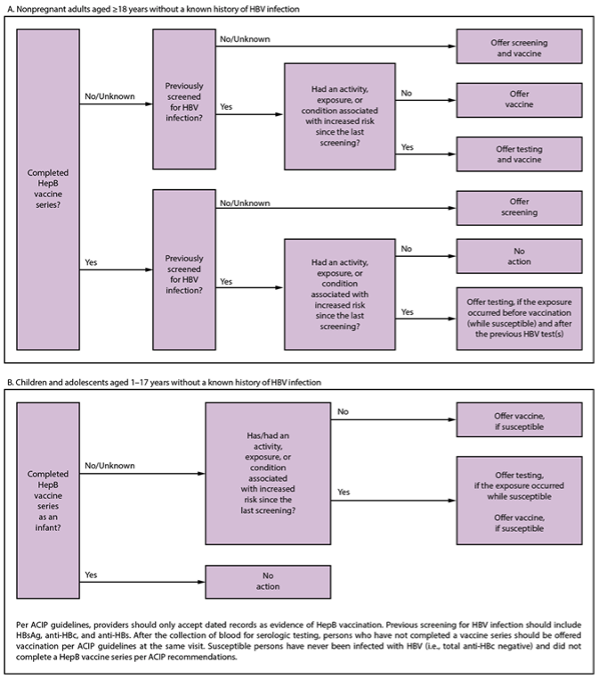
Incorporating Hepatitis B Virus Screening and Testing into Clinic Workflow
Below is a flowchart containing CDC guidance for clinics or physicians to use to screen and test peeople without a known history of HBV infection, including non-pregnant adult patients, ages 18 years and older, as well as children and adolescents, ages 1 to 17 years old.
Resources
Communication Materials
A Pediatric Guide: Caring for Infants Born to Hepatitis B-Infected Mothers
Updated December 24, 2025, 11:56 AM